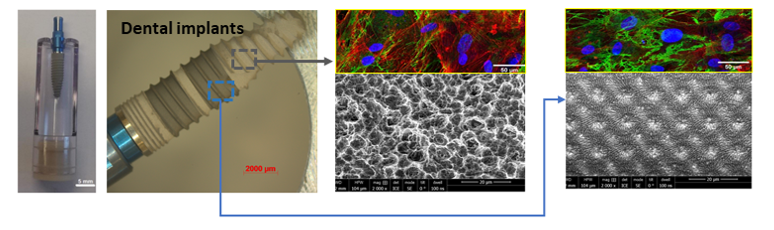
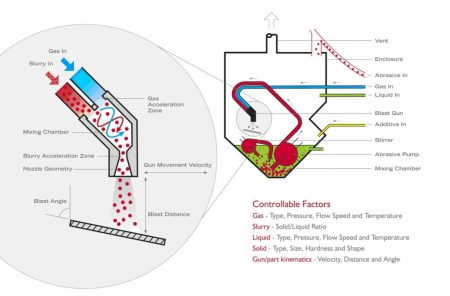
Based in Dijon (composed of 80 people with a 2,100 m² laboratory), we are ISO 17025 accredited for cleaning residue analysis according to ISO 19227: 2018, as well as for chemical characterization studies of materials according to ISO 10993-18, ISO 10993-13, ISO 10993-14, ISO 10993-19 et ISO 10993-22.
(Scopes available on www.cofrac.fr – Accreditation number: 1-1793.)
OUR SERVICES :
We provide daily support to our industrial medical device customers, including orthopedic implants, for their needs:
Chemical analyzes
- cleaning residue analysis according to ISO 19227: analysis of organic, inorganic contaminants and particulate contamination
- undesirable substance analyzes (CMR, etc.)
- sterilization residue analyzes (ISO 10993-7)
- development of new analysis methods
Assessing the biocompatibility of medical devices
- chemical characterization of materials according to ISO 10993-18
- study of degradation products according to ISO 10993-13 and ISO 10993-14
- PMT characterization according to ISO 10993-19
- characterization of nanomaterials according to ISO 10993-22
Chemical characterization of materials according to ISO 10993-18: 2020
- analyzes of ceramics and hydroxyapatite (HAP) powders according to ISO 13779:2018
- characterization of polymer materials (silicones, resins, elastomers, etc.)
- chemical analyzes of metal alloys, determination of grades
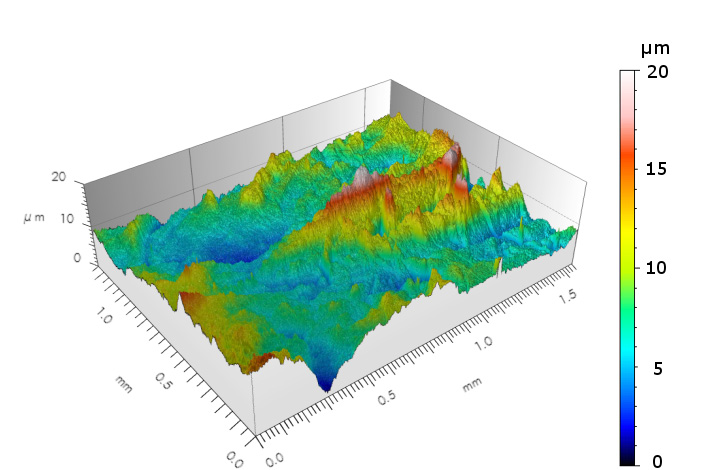
- extreme surface characterization of materialsI
Expertise and problem solving
- search and identification of surface contaminations
- chemical treatment problem (galvanization, passivation, anodization…)
- breakage, cracking of parts
- corrosion phenomenon
- surface treatment detachment
- industrial process audit
WHY FILAB?
- An experienced and accessible team
- An ISO 17025 accredited laboratory
- A complete 2100m² analytical fleet
- Competitive deadlines and prices